Briefly Explain the Difference Between Cuso4 S and Cuso4 Aq
CuSO4s and CuSO4 x 5H20s b. Rep gems come when your posts are rated by other community members.
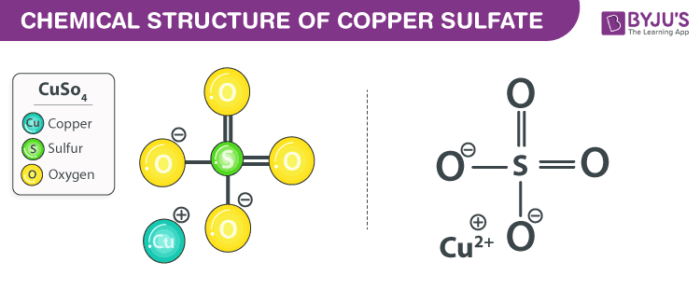
Cuso4 Aq Zn S Cu S Znso4 Aq Is An Example Of Chemistry Q A
Solubility of CuSO4 at 60C in 100 mL H2O is 40 g and in 0C is 15g.

. In a single displacement reaction between a metal and an aqueous solution of a strong acid such as Zn s 2 HCl aq ZnCl2 aq H2 g gas bubbles are evidence of a reaction. Once the reaction is complete and there is no more CuSO4 present in the solution it will appear completely colorless. What is the difference between CuSO 4 s and CuSO 4 aq.
CuSO 4 aq Zn s Cu s ZnSO 4 aq is an example of displacement reaction. This becomes whitish when anhydrous when it is not molecularly bound to water. The ratio of combination is 114 that is one atom of copper combine with one atom of sulphur and four atoms of oxygen to form one molecule of CUSO4.
CuSO4s in water Cu2aq SO42aq Sulphate ion SO42aq stays the same. According to the question there will be two situations. Soln of CuSO4 25g dissolved in min amnt of water at 60C then cooled at 0C.
If this is observed Zn s was oxidized. CuSO4s Zns - Cus ZnSO4aq 322 g of powdered zinc is added to 200ml of 05M copper sulfate solution. The oxidation number of an atom in its elemental form is ___.
5H O s and CuSO aq. Cupric sulfate is a salt created by treating cupric oxide with sulfuric acidThis forms as large bright blue crystals containing five molecules of water CuSO45H2O and is also known as blue vitriolThe anhydrous salt is created by heating the hydrate to 150 C 300 F. How much CuSo45H2O will precipitate from a aq.
Answer 1 of 2. Mgs 2H2l --- MgOH2aq H2g Mgl 2H2Ol --- MgOH2aq H2g. Laminiaduo7 and 10 more users found this answer helpful.
What mass of copper is deposited. But there can be varying number of water molecules or sometimes no water molecules in the compound. Often CuSO 4 s is a blue color crystal.
CuSO4s and CuSO4 x 5H20s b. CuSO4s and CuSO4aq Question. Chemical Reactions of Copper and Percent Yield.
The two chemical equations below appear to describe the same chemical reaction. Initial mass of copper 2. Copper II sulfate is a hydrated blue solid it is attached to water molecules.
But CuSO 4 aq is a blue color solution. Cuso4 is a water absorbent agent. Cuso4 crystals are white.
And when you hear the cuso4 water evaporates and blue turns into white. Explain how the two reactions are different form each other. In the question given above CUSO4 is a compound because copper sulphur and oxgyen had combined together chemically to form a new compound which is CUSO4.
See the answer See the answer See the answer done loading. When it is hydrated there are usually five molecules of water attached to one cooper sulphate molecule. Zns CuSO4aq -- Cus ZnSO4aq Using our ending solution from the last reaction we carefully weighed out about 2 grams of mossy zinc to add.
5H O S 4 4 2. Cupric sulfate is used primarily for agricultural purposes as a pesticide germicide feed additive and soil additive. Posted on October 8 2020 by Joseph.
Mass of evaporating dish 4. There are several physical indicators of the reaction between iron and CuSO4. Often in CuSO 4 s there are five water molecules.
Hypothesize and differences you might be able to observe in these two reactions represented by the following equations. However cupriccopperII ion Cu2aq reacts with water to form hexaaquacopperII ions CuH2O62aq It is this ion that gives the solution blue. Explain the difference between the following formulas.
Because when cuso4 is exposed to the atmosphere it absorbs water vapor present in the air. In fact without this water CuSO4s would be colourless white Without the water CuSO4s is described as anhydrous without water If you dissolve either hydrated CuSO4 or anhydrous CuSO4 in water the ions break up and the salt become aqueous aq and can be written as CuSO4aq. Heating up the CuSO4 will dehydrate it.
CuSO4 is an anhydrous salt which will absorb water so the way to find out how much is in it is to find out. Usually we can see cuso4 is blue in color. Okay so I know how I would normally determine molar mass of a compound get out periodic table sum up elements present eg.
CuSO4s and CuSO4aq This problem has been solved. In the above reaction Zn is added to the solution of copper sulphate and Zn displaces Cu from its salt solution. Report 18 years ago.
2 CuSO s and CuSO aq 4 4. The result of adding the zinc caused fizzing color change of the zinc black-red and resulted in copper formation. Explain the difference between the following formulas.
A displacement reaction is a chemical reaction in which a more reactive element displaces a less reactive element from its compound. Percent yield show calculations 6. Chemists use symbolism to facilitate writing of chemical equations.
Briefly explain the difference between. 1 CuSO s and CuSO. Mass of recovered copper 5.
Cuso45h2o crystals are blue. Aqueous CuSO4 is a blue solution and it will lose its color as it reacts with the iron to form FeSO4. Mass of copper and evaporating dish 3.
Youll earn badges for being active around the site. 1wet cuso4 blue 2dry cuso4 white.

Difference Between Cuso4 And Cuso4 5h2o Compare The Difference Between Similar Terms

Perbedaan Antara Cuso4 Dan Cuso4 5h2o Bandingkan Perbedaan Antara Istilah Serupa Ilmu 2022
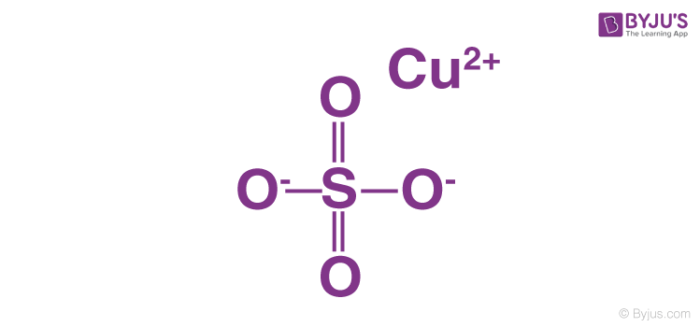
Copper Sulfate Structure Properties And Uses Of Cuso4

Perbedaan Antara Cuso4 Dan Cuso4 5h2o Bandingkan Perbedaan Antara Istilah Serupa Ilmu 2022
No comments for "Briefly Explain the Difference Between Cuso4 S and Cuso4 Aq"
Post a Comment